Difference between revisions of "Isotope"
From Linked Earth Wiki
(Add information about the standard delta notation) |
m (Change anchor place) |
||
| Line 1: | Line 1: | ||
Atoms can be simply described as consisting of [https://en.wikipedia.org/wiki/Proton protons], [https://en.wikipedia.org/wiki/Electron electrons], and [https://en.wikipedia.org/wiki/Neutron neutrons]. Isotopes of the same element differ by the number of neutrons in the nucleus, resulting in different mass. | Atoms can be simply described as consisting of [https://en.wikipedia.org/wiki/Proton protons], [https://en.wikipedia.org/wiki/Electron electrons], and [https://en.wikipedia.org/wiki/Neutron neutrons]. Isotopes of the same element differ by the number of neutrons in the nucleus, resulting in different mass. | ||
| − | ==<div id="delta_notation"> | + | ==The δ-notation== |
| + | |||
| + | <div id="delta_notation"></div> | ||
Because relative differences in isotope ratios are more precisely detected than the absolute isotopic ratios, they are commonly reported in the δ-notation: | Because relative differences in isotope ratios are more precisely detected than the absolute isotopic ratios, they are commonly reported in the δ-notation: | ||
Latest revision as of 15:06, 15 January 2016
Atoms can be simply described as consisting of protons, electrons, and neutrons. Isotopes of the same element differ by the number of neutrons in the nucleus, resulting in different mass.
The δ-notation
Because relative differences in isotope ratios are more precisely detected than the absolute isotopic ratios, they are commonly reported in the δ-notation:
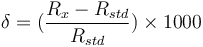
where 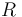 is the ratio of the abundance of the heavy to the light isotope,
is the ratio of the abundance of the heavy to the light isotope, 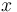 is the measured sample, and
is the measured sample, and 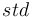 is the standard. For the element oxygen,
is the standard. For the element oxygen, 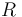 is given by 18O/16O.
is given by 18O/16O.