Difference between revisions of "Stable oxygen isotopes in foraminifera"
m (Link stable oxygen isotopes to the internal page) |
m (Proper references added) |
||
| Line 1: | Line 1: | ||
| − | The fractionation of [[Stable oxygen isotopes | oxygen isotopes]] between [https://en.wikipedia.org/wiki/Water water] and [https://en.wikipedia.org/wiki/Calcium_carbonate calcium carbonate] is temperature sensitive, which prompted [https://en.wikipedia.org/wiki/Harold_Urey H.C. Urey] in 1947 to propose that this phenomenon could be used as a [https://en.wikipedia.org/wiki/Paleothermometer paleothermometer]. | + | The fractionation of [[Stable oxygen isotopes | oxygen isotopes]] between [https://en.wikipedia.org/wiki/Water water] and [https://en.wikipedia.org/wiki/Calcium_carbonate calcium carbonate] is temperature sensitive, which prompted [https://en.wikipedia.org/wiki/Harold_Urey H.C. Urey] in 1947 <ref>Urey, H. C., Epstein, S., & McKinney, C. R. (1948). Method for measurements of paleotemperatures. Bulletin of the Geological Society of America (abstract), 59, 1359-1360. </ref> to propose that this phenomenon could be used as a [https://en.wikipedia.org/wiki/Paleothermometer paleothermometer]. |
| − | The oxygen isotopic composition of a carbonate samples is measured by [https://en.wikipedia.org/wiki/Mass_spectrometry mass spectrometry] using CO<sub>2</sub> gas released when carbonate samples are treated with 100% [https://en.wikipedia.org/wiki/Phosphoric_acid phosphoric acid] in an evacuated vessel at a controlled temperature, typically 90°C | + | The oxygen isotopic composition of a carbonate samples is measured by [https://en.wikipedia.org/wiki/Mass_spectrometry mass spectrometry] using CO<sub>2</sub> gas released when carbonate samples are treated with 100% [https://en.wikipedia.org/wiki/Phosphoric_acid phosphoric acid] in an evacuated vessel at a controlled temperature, typically 90°C <ref> McCrea, J. M. (1950). On the isotopic chemistry of carbonates and a paleotemperature scale. Journal of Chemical Physics, 18, 849-857.</ref>. The stable oxygen isotope rations are usually expressed using the δ-notation referenced to the Vienna Pee Dee Belemnite (VPDB). |
| − | The temperature dependence of the fractionation had been determined by several groups of investigators and has been expressed in different forms. The classic temperature equation derived by Epstein et al. (1953) takes the form: | + | The temperature dependence of the fractionation had been determined by several groups of investigators and has been expressed in different forms. The classic temperature equation derived by Epstein et al. (1953) ,<ref>Epstein, S., Buchsbaum, R., Lowenstam, H. A., & Urey, H. C. (1953). Revised carbonate-water isotopic temperature scale. Geological Society of America Bulletin, 64, 1315-1326. |
| + | </ref> takes the form: | ||
<math> T(^\circ C)=16.9-4.2(\delta^{18}O_{c}-\delta^{18}O_{sw(VSMOW)})+0.13(\delta^{18}O_{c}-\delta^{18}O_{sw(VSMOW)})^2</math> | <math> T(^\circ C)=16.9-4.2(\delta^{18}O_{c}-\delta^{18}O_{sw(VSMOW)})+0.13(\delta^{18}O_{c}-\delta^{18}O_{sw(VSMOW)})^2</math> | ||
| Line 9: | Line 10: | ||
where δ<sup>18</sup>O<sub>sw</sub> represents the stable oxygen isotopic composition of seawater in which the carbonate [https://en.wikipedia.org/wiki/Precipitation_(chemistry) precipitated]. | where δ<sup>18</sup>O<sub>sw</sub> represents the stable oxygen isotopic composition of seawater in which the carbonate [https://en.wikipedia.org/wiki/Precipitation_(chemistry) precipitated]. | ||
| − | However, paleothermometry based on the temperature dependence of the fractionation of oxygen isotopes between marine carbonates and water must satisfy a set of assumptions concerning: (1) the existence of isotopic equilibrium between oxygen in the water and in biogenic calcite of the presence of "vital effects", (2) the preservation of the isotope composition of oxygen in the solid carbonate shell as subsequent [https://en.wikipedia.org/wiki/Dissolution_(chemistry) dissolution] and/or replacement by calcium carbonate that equilibrated at a different temperature with water having a different isotopic composition may occur, (3) the presence of crystals of [https://en.wikipedia.org/wiki/Diagenesis diagenetic] calcite in foraminiferal shells, whose oxygen isotope composition is difference from that of the shell, and (4) the constancy of the isotope composition of water in the oceans ( | + | However, paleothermometry based on the temperature dependence of the fractionation of oxygen isotopes between marine carbonates and water must satisfy a set of assumptions concerning: (1) the existence of isotopic equilibrium between oxygen in the water and in biogenic calcite of the presence of "vital effects", (2) the preservation of the isotope composition of oxygen in the solid carbonate shell as subsequent [https://en.wikipedia.org/wiki/Dissolution_(chemistry) dissolution] and/or replacement by calcium carbonate that equilibrated at a different temperature with water having a different isotopic composition may occur, (3) the presence of crystals of [https://en.wikipedia.org/wiki/Diagenesis diagenetic] calcite in foraminiferal shells, whose oxygen isotope composition is difference from that of the shell, and (4) the constancy of the isotope composition of water in the oceans <ref name="sharp2007"> Sharp, Z. (2007). Principles of stable isotope geochemistry. Upper Saddle River, New Jersey: Pearson Prentice Hall. </ref>. |
| − | Violating these assumptions can limit the use δ<sup>18</sup>O of [https://en.wikipedia.org/wiki/Biogenic_substance biogenic] calcite as a true paleothermometer. Planktonic foraminifera tend to secrete their shells in isotopic equilibrium with seawater. However, divergence from equilibrium, termed "vital effects", has been observed and is related to [https://en.wikipedia.org/wiki/Environment_(biophysical) environmental] factors such as sunlight intensity, temperature stress, and nutrient supply | + | Violating these assumptions can limit the use δ<sup>18</sup>O of [https://en.wikipedia.org/wiki/Biogenic_substance biogenic] calcite as a true paleothermometer. Planktonic foraminifera tend to secrete their shells in isotopic equilibrium with seawater. However, divergence from equilibrium, termed "vital effects", has been observed and is related to [https://en.wikipedia.org/wiki/Environment_(biophysical) environmental] factors such as sunlight intensity, temperature stress, and nutrient supply <ref name= "sharp2007" />. This had led to species-specific calibration equation <ref name="bemis1998"> Bemis, B. E., Spero, H. J., Bijma, J., & Lea, D. W. (1998). Reevaluation of the oxygen isotopic composition of planktonic foraminifera: Experimental results and revised paleotemperature equations. Paleoceanography, 13(2), 150-160. </ref>. |
Oxygen isotope rations of biogenic calcite can be changed by two diagenetic processes: (1) the addition of new carbonate by cementation, and (2) the dissolution of unstable carbonate and the re-precipitation of a stable mineral. Great care is taken to avoid the effects of diagenesis, but there is no foolproof method to prove that no diagenesis has taken place. | Oxygen isotope rations of biogenic calcite can be changed by two diagenetic processes: (1) the addition of new carbonate by cementation, and (2) the dissolution of unstable carbonate and the re-precipitation of a stable mineral. Great care is taken to avoid the effects of diagenesis, but there is no foolproof method to prove that no diagenesis has taken place. | ||
| − | The stable oxygen isotopic composition of surface waters is influenced by [https://en.wikipedia.org/wiki/Evaporation evaporation], [https://en.wikipedia.org/wiki/Precipitation precipitation], [https://en.wikipedia.org/wiki/Mixing_(physics) mixing] and [https://en.wikipedia.org/wiki/River river] runoff. These processes also control [https://en.wikipedia.org/wiki/Salinity sea surface salinity], and therefore, there is a strong linear relationship between local salinity and δ<sup>18</sup>O<sub>sw</sub> | + | The stable oxygen isotopic composition of surface waters is influenced by [https://en.wikipedia.org/wiki/Evaporation evaporation], [https://en.wikipedia.org/wiki/Precipitation precipitation], [https://en.wikipedia.org/wiki/Mixing_(physics) mixing] and [https://en.wikipedia.org/wiki/River river] runoff. These processes also control [https://en.wikipedia.org/wiki/Salinity sea surface salinity], and therefore, there is a strong linear relationship between local salinity and δ<sup>18</sup>O<sub>sw</sub> <ref> LeGrande, A. N., & Schmidt, G. A. (2006). Global gridded data set of the oxygen isotopic composition in seawater. Geophysical Research Letters, 33, L12604. doi:doi:10.1029/2006GL026011 </ref>. On longer time scales, the δ<sup>18</sup>O<sub>sw</sub> of the entire ocean is also controlled by ice volume changes that accompany the glacial-interglacial cycles. During glacials, the δ<sup>18</sup>O<sub>sw</sub> increases when isotopically light water is transferred from the ocean to the continental ice sheets. Therefore, an estimation of paleo-salinity and ice volume changes must be made in order to use δ<sup>18</sup>O<sub>calcite</sub> as a paleothermometer. Conversely, if the temperature at which the biogenic calcite precipitated can be estimated through other means, then the δ<sup>18</sup>O<sub>calcite</sub> can be used to infer past changes in δ<sup>18</sup>O<sub>sw</sub>. |
| + | |||
| + | ==References== | ||
| + | <references \> | ||
Revision as of 17:57, 15 January 2016
The fractionation of oxygen isotopes between water and calcium carbonate is temperature sensitive, which prompted H.C. Urey in 1947 [1] to propose that this phenomenon could be used as a paleothermometer.
The oxygen isotopic composition of a carbonate samples is measured by mass spectrometry using CO2 gas released when carbonate samples are treated with 100% phosphoric acid in an evacuated vessel at a controlled temperature, typically 90°C [2]. The stable oxygen isotope rations are usually expressed using the δ-notation referenced to the Vienna Pee Dee Belemnite (VPDB).
The temperature dependence of the fractionation had been determined by several groups of investigators and has been expressed in different forms. The classic temperature equation derived by Epstein et al. (1953) ,[3] takes the form:
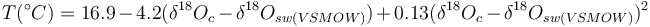
where δ18Osw represents the stable oxygen isotopic composition of seawater in which the carbonate precipitated.
However, paleothermometry based on the temperature dependence of the fractionation of oxygen isotopes between marine carbonates and water must satisfy a set of assumptions concerning: (1) the existence of isotopic equilibrium between oxygen in the water and in biogenic calcite of the presence of "vital effects", (2) the preservation of the isotope composition of oxygen in the solid carbonate shell as subsequent dissolution and/or replacement by calcium carbonate that equilibrated at a different temperature with water having a different isotopic composition may occur, (3) the presence of crystals of diagenetic calcite in foraminiferal shells, whose oxygen isotope composition is difference from that of the shell, and (4) the constancy of the isotope composition of water in the oceans [4].
Violating these assumptions can limit the use δ18O of biogenic calcite as a true paleothermometer. Planktonic foraminifera tend to secrete their shells in isotopic equilibrium with seawater. However, divergence from equilibrium, termed "vital effects", has been observed and is related to environmental factors such as sunlight intensity, temperature stress, and nutrient supply [4]. This had led to species-specific calibration equation [5].
Oxygen isotope rations of biogenic calcite can be changed by two diagenetic processes: (1) the addition of new carbonate by cementation, and (2) the dissolution of unstable carbonate and the re-precipitation of a stable mineral. Great care is taken to avoid the effects of diagenesis, but there is no foolproof method to prove that no diagenesis has taken place.
The stable oxygen isotopic composition of surface waters is influenced by evaporation, precipitation, mixing and river runoff. These processes also control sea surface salinity, and therefore, there is a strong linear relationship between local salinity and δ18Osw [6]. On longer time scales, the δ18Osw of the entire ocean is also controlled by ice volume changes that accompany the glacial-interglacial cycles. During glacials, the δ18Osw increases when isotopically light water is transferred from the ocean to the continental ice sheets. Therefore, an estimation of paleo-salinity and ice volume changes must be made in order to use δ18Ocalcite as a paleothermometer. Conversely, if the temperature at which the biogenic calcite precipitated can be estimated through other means, then the δ18Ocalcite can be used to infer past changes in δ18Osw.
References
- ↑ Urey, H. C., Epstein, S., & McKinney, C. R. (1948). Method for measurements of paleotemperatures. Bulletin of the Geological Society of America (abstract), 59, 1359-1360.
- ↑ McCrea, J. M. (1950). On the isotopic chemistry of carbonates and a paleotemperature scale. Journal of Chemical Physics, 18, 849-857.
- ↑ Epstein, S., Buchsbaum, R., Lowenstam, H. A., & Urey, H. C. (1953). Revised carbonate-water isotopic temperature scale. Geological Society of America Bulletin, 64, 1315-1326.
- ↑ 4.0 4.1 Sharp, Z. (2007). Principles of stable isotope geochemistry. Upper Saddle River, New Jersey: Pearson Prentice Hall.
- ↑ Bemis, B. E., Spero, H. J., Bijma, J., & Lea, D. W. (1998). Reevaluation of the oxygen isotopic composition of planktonic foraminifera: Experimental results and revised paleotemperature equations. Paleoceanography, 13(2), 150-160.
- ↑ LeGrande, A. N., & Schmidt, G. A. (2006). Global gridded data set of the oxygen isotopic composition in seawater. Geophysical Research Letters, 33, L12604. doi:doi:10.1029/2006GL026011